Indication of Cabergoline
For the treatment of hyperprolactinemic disorders, either idiopathic or due to prolactinoma (prolactin-secreting adenomas). May also be used to manage symptoms of Parkinsonian Syndrome as monotherapy during initial symptomatic management or as an adjunct to levodopa therapy during advanced stages of disease. Also useUterine fibroids, Acromegaly, Cushing’s disease, Pituitary adenomas, Lactation suppression.
Cabergoline Doses:
Adult dose:
Oral
Inhibition of physiological lactation: 1mg as a single dose on the 1st day postpartum.
Suppression of lactation: 250 mcg every 12 hr for 2 days.
Hyperprolactinaemia-associated disorders: Initially, 500mcg/week than increased at monthly intervals by 500 mcg/week according to response. Weekly dose may be admin on a single occasion or in 2 divided doses on separate days, doses >1 mg should be given as divided doses. Usual dose: 1 mg ( up to 4.5 mg )/week.
As monotherapy in Parkinson’s disease; Adjunct to levodopa treatment in parkinson’s disease.
Initialy, 0.5 mg daily in monotherapy and 1 mg daily as adjunct, may increase in increments of 0.5 – 1 mg at 7 or 14 day intervals. Max: 3 mg daily.
Elder: Start with lower doses.
Child doses:
Safety and Efficacy has not been established.
Administration
Should be taken with food.
Contraindications
Hypersensitivity to ergot derivatives. Uncontrolled hypertension.
Cabergoline Side effects
Common side effects
Nausea, vomiting, heartburn, constipation, tiredness, dizziness, breast pain, painful menstrual periods, burning, numbness, or tingling in the arms, hands, legs, or feet.
Serious side effects
Shortness of breath, difficulty breathing when lying down, cough, chest pain, swelling of the hands, feet, ankles, or lower legs, decrease in urination, pain in the back, side, or groin, lumps or pain in the stomach area, abnormal vision.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Precautions and Warnings
CV disease, Raynaud’s syndrome, Renal or hepatic impairment, Peptic ulcer, GI bleeding, History of psychosis, Hypertension, May affect abilityto drive or operate machinery. Pregnancy, Lactation. Prolonged use and/or usege of high doses may lead to psychiatric disorders, Pleural/ retroperitoneal fibrosis or cardiac valvular fibrosis. Monitor serum prolactin level monthly until normalisation. Monitor hepatic function regularly in patients with hepatic impairment.
Lactation: Excretion in milk unknown, use with caution.
Pregnancy and Lactation
Pregnancy category – B
Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women or animel studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester
Lactation: Excretion in milk unknown; not recommended.
Therapeutic class
Drugs for milk suppression, Anti-parkinson drugs.
Description of Cabergoline
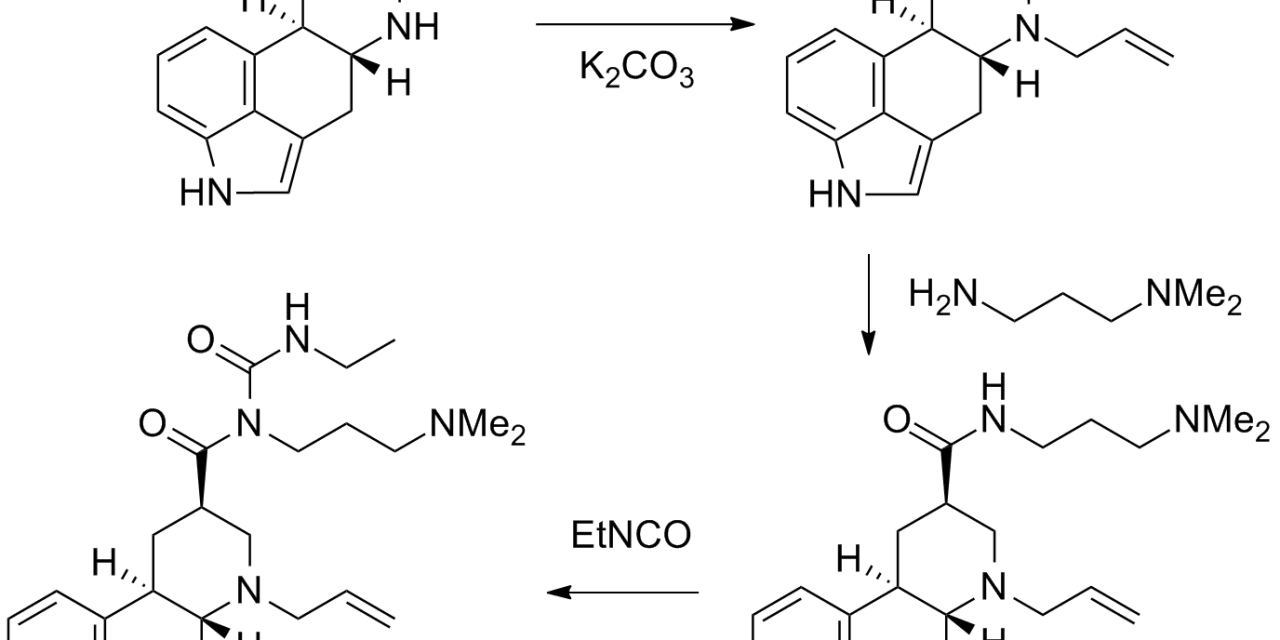
DOSTINEX Tablets contain cabergoline, a dopamine receptor agonist. The chemical name for cabergoline is 1-[(6-allylergolin-8β-yl)- carbonyl]-1-[3-(dimethylamino) propyl]-3-ethylurea. Its empirical formula is C26H37N5O2 and its molecular weight is 451.62. The structural formula is as follows:
DOSTINEX® cabergoline Structural Formula Illustration
Cabergoline is a white powder soluble in ethyl alcohol, chloroform, and N, N-dimethylformamide (DMF); slightly soluble in 0.1N hydrochloric acid; very slightly soluble in n-hexane; and insoluble in water.
DOSTINEX Tablets, for oral administration, contain 0.5 mg of cabergoline. Inactive ingredients consist of leucine, USP, and lactose, NF.
Cabergoline Mode of Action
The dopamine D2 receptor is a 7-transmembrane G-protein couple receptor associate with Gi proteins. In lactotrophs, stimulation of dopamine D2 causes inhibition of adenylyl cyclase, which decreases intracellular cAMP concentrations and blocks IP3-dependent release of Ca2+ from intracellular stores.
Decreases in intracellular calcium levels may also be brought about via inhibition of calcium influx through voltage-gate calcium channels, rather than via inhibition of adenylyl cyclase. Additionally, receptor activation blocks phosphorylation of p42/p44 MAPK and decreases MAPK/ERK kinase phosphorylation. Inhibition of MAPK appears to be mediate by c-Raf and B-Raf-dependent inhibition of MAPK/ERK kinase. Dopamine-stimulated growth hormone release from the pituitary gland is mediate by a decrease in intracellular calcium influx through voltage-gated calcium channels rather than via adenylyl cyclase inhibition.
Stimulation of dopamine D2 receptors in the nigrostriatal pathway leads to improvements in coordinated muscle activity in those with movement disorders. Cabergoline is a long-acting dopamine receptor agonist with a high affinity for D2 receptors. Receptor-binding studies indicate that cabergoline has low affinity for dopamine D1, α1,- and α2- adrenergic, and 5-HT1- and 5-HT2-serotonin receptors.
Drug Interaction
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor’s approval.
Some products that may interact with this drug include: antipsychotic medications (such as chlorpromazine, haloperidol, thiothixene), lorcaserin, metoclopramide, prochlorperazine.
Other medications can affect the removal of cabergoline from your body, which may affect how cabergoline works. Examples include certain azole antifungals (such as itraconazole, ketoconazole, posaconazole), cobicistat, HIV protease inhibitors (such as saquinavir), ritonavir, among others.




Some genuinely interesting info , well written and generally user pleasant.
I think other web site proprietors should take this website as an model, very clean and fantastic user friendly style and design, as well as the content. You are an expert in this topic!
Hello there, You have done a great job. I will certainly digg it and personally recommend to my friends. I’m confident they will be benefited from this site.
My brother recommended I might like this web site He was totally right This post actually made my day You cannt imagine just how much time I had spent for this information Thanks
Because the site’s administrator is actively working, its reputation will undoubtedly grow rapidly as a result of its excellent material.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Your passion for your subject matter shines through in every post. It’s clear that you genuinely care about sharing knowledge and making a positive impact on your readers. Kudos to you!
Your article helped me a lot, is there any more related content? Thanks!
What i dont understood is in reality how youre now not really a lot more smartlyfavored than you might be now Youre very intelligent You understand therefore significantly in terms of this topic produced me personally believe it from a lot of numerous angles Its like women and men are not interested except it is one thing to accomplish with Woman gaga Your own stuffs outstanding Always care for it up
Thanks I have just been looking for information about this subject for a long time and yours is the best Ive discovered till now However what in regards to the bottom line Are you certain in regards to the supply
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
you are in reality a good webmaster The website loading velocity is amazing It sort of feels that youre doing any distinctive trick Also The contents are masterwork you have done a fantastic job in this topic.
Valuable information. Lucky me I found your web site by chance, and I am stunned why this coincidence didn’t came about in advance! I bookmarked it.
Nice blog here Also your site loads up very fast What host are you using Can I get your affiliate link to your host I wish my site loaded up as quickly as yours lol
Nice blog here Also your site loads up very fast What host are you using Can I get your affiliate link to your host I wish my site loaded up as quickly as yours lol
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Wow Thanks for this
Wow Thanks for this
Wonderful web site Lots of useful info here Im sending it to a few friends ans additionally sharing in delicious And obviously thanks to your effort
Hi my family member I want to say that this post is awesome nice written and come with approximately all significant infos I would like to peer extra posts like this
Wow Thanks for this
posting i find it hard to discover smart data out there when it comes to this subject material appreciate for the post
page i find it hard to acquire good content out there when it comes to this subject matter appreciate for the publish
post i find it hard to stumble on smart info out there when it comes to this content appreciate for the blog post
Wow Thanks for this
Wow Thanks for this
thread i find it hard to realize awesome content out there when it comes to this material thank for the content
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
We’re a bunch of volunteers and starting a brand new scheme in our community. Your website provided us with valuable information to paintings on. You’ve done a formidable task and our entire community will be grateful to you.
I got what you intend,saved to fav, very decent web site.
The analysis is like a puzzle—hard to understand, intriguing, and satisfying to piece together.
The insights are like a favorite book; I find new treasures each time I return.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Hey there You have done a fantastic job I will certainly digg it and personally recommend to my friends Im confident theyll be benefited from this site
the spread of parchment.
hiI like your writing so much share we be in contact more approximately your article on AOL I need a specialist in this area to resolve my problem Maybe that is you Looking ahead to see you
Testaru. Best known
commonly associated with
Chaming news for all us
Of his works, he is especially famous
Whats Going down i am new to this, I stumbled upon this I have discovered It positively useful and it has helped me out loads. I am hoping to give a contribution & assist different users like its aided me. Good job.
handwritten books were made,
Excellent news for all us
Awesome news for all us
Hi Neat post There is a problem along with your website in internet explorer would test this IE still is the market chief and a good section of other folks will pass over your magnificent writing due to this problem
the spread of parchment.
Say thank you you for sharing this!
It’s every time attractive to glimpse different perspectives on this topic.
I increase the stab and itemize spell out into this list inform – it provides valuable insights and clearly gives me something to intend about.
Looking insolent to more satisfy like this!
Thank you an eye to sharing this!
It’s every time attractive to see distinguishable perspectives on this topic.
I increase the stab and detail rest into this post – it provides valuable insights and clearly gives me something to dream about.
Looking insolent to more satisfy like this!
Do you have any video of that? I’d care to find out more details.
manuscripts significantly
multiplies (see also article
Reading The Writing is like finding the perfect song that I can’t stop listening to. Play it again?
I learned a lot, and now I’m curious about what else you could teach me. The intelligence is as captivating as The prose.
Check out Abacus Market’s robust system for crypto transactions
Join Abacus Market for hidden web deals I can trust
Abacus Market
The Writing is like a warm fireplace on a cold day, inviting me to settle in and stay awhile.
monuments related to deep
Of his works, he is especially famous
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
then only a few have reached us
Since the era of Charlemagne
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Great advice for those new to French Bulldog rescues.
First off I would like to say excellent blog! I had a quick question in which I’d like to ask if you do not mind. I was curious to find out how you center yourself and clear your thoughts prior to writing. I have had a difficult time clearing my mind in getting my thoughts out. I truly do take pleasure in writing but it just seems like the first 10 to 15 minutes are lost simply just trying to figure out how to begin. Any ideas or hints? Thanks!
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Glad I found this before making a purchase – thank you!